Description
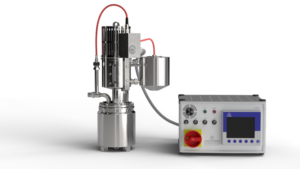
A distiller is used for distillation. Distillation is a thermal separation process for obtaining vaporizable liquids or separating solvents from substances that are difficult to vaporize and then collecting them by condensation. Compared to other separation processes, distillation has the advantage that, as a rule, no…
A distiller is used for distillation. Distillation is a thermal separation process for obtaining vaporizable liquids or separating solvents from…
Suppliers
The following suppliers offer Distiller products.
-
Anton Paar Switzerland AG
-
Avantor – VWR International GmbH
-
Faust Laborbedarf AG
-
Haslab GmbH
-
Huberlab AG
-
IKA-Werke GmbH & Co. KG
-
JUCHHEIM Laborgeräte GmbH
-
Milian SA
-
Renggli AG
-
Vitaris AG